Embark on a journey into the fascinating world of acids and bases with our Acids and Bases Review Worksheet. This comprehensive guide unravels the complexities of these chemical substances, providing a thorough understanding of their properties, reactions, and applications.
Acids and bases play a crucial role in our daily lives, from the batteries that power our devices to the cleaning products we use in our homes. Delve into the depths of this captivating topic and discover the hidden secrets of acids and bases.
Introduction
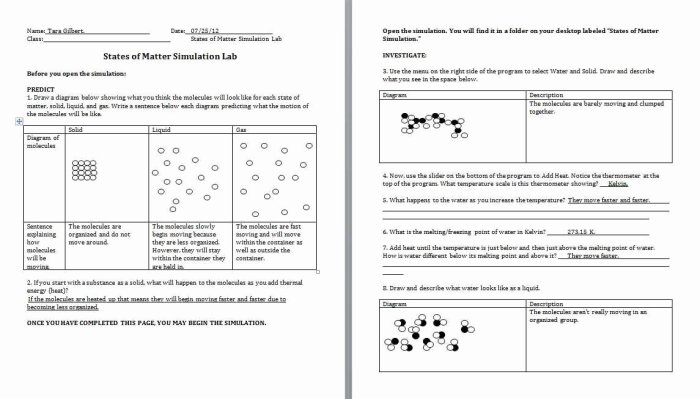
Acids and bases are essential concepts in chemistry that help us understand a wide range of chemical reactions and processes in our world. They play a crucial role in various industries, such as food processing, medicine, and manufacturing. By comprehending the properties and behaviors of acids and bases, we gain insights into the interactions between different substances and can better predict their outcomes.
Overview of Acids and Bases
Acids are chemical compounds that release hydrogen ions (H+) when dissolved in water. They typically taste sour, react with metals to produce hydrogen gas, and turn blue litmus paper red. Bases, on the other hand, release hydroxide ions (OH-) when dissolved in water.
They have a bitter taste, feel slippery to the touch, and turn red litmus paper blue. The strength of an acid or base is determined by its dissociation constant, which measures the extent to which it ionizes in water.
Properties of Acids and Bases
Acids and bases exhibit distinct properties that allow us to identify and distinguish them. These properties are crucial in understanding their behavior and applications in various fields.
Characteristic Properties of Acids
Acids are characterized by several notable properties:
- Sour Taste:Acids have a characteristic sour taste, which is familiar from substances like vinegar or lemon juice.
- Reactivity with Metals:Acids react with certain metals, such as zinc or iron, to produce hydrogen gas. This reaction is often accompanied by the formation of a salt.
- Litmus Paper Test:Acids turn blue litmus paper red, indicating their acidic nature.
Characteristic Properties of Bases
Bases, on the other hand, exhibit the following properties:
- Bitter Taste:Bases have a bitter taste, which can be experienced in substances like soap or baking soda.
- Slippery Feel:Bases feel slippery to the touch, as they tend to react with oils on the skin, forming soap-like substances.
- Litmus Paper Test:Bases turn red litmus paper blue, signaling their basic nature.
Understanding these characteristic properties is essential for recognizing and handling acids and bases safely and effectively.
pH Scale
The pH scale is a measure of how acidic or basic a solution is. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while those with a pH above 7 are basic.
The pH scale is logarithmic, meaning that each whole number change in pH represents a tenfold change in acidity or basicity. For example, a solution with a pH of 6 is ten times more acidic than a solution with a pH of 7, and a solution with a pH of 8 is ten times more basic than a solution with a pH of 7.
Using the pH Scale
The pH scale is used to classify solutions as acidic, neutral, or basic. A solution with a pH below 7 is acidic, a solution with a pH of 7 is neutral, and a solution with a pH above 7 is basic.
The pH scale is also used to determine the strength of acids and bases. A strong acid has a low pH, while a weak acid has a high pH. Similarly, a strong base has a high pH, while a weak base has a low pH.
Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of protons (H+) between acids and bases. These reactions are essential in many chemical processes, such as digestion, respiration, and the regulation of pH in the body.
There are three main types of acid-base reactions:
Neutralization Reactions
Neutralization reactions occur when an acid and a base react in equal molar amounts to form a salt and water. The salt is a compound that contains the cation from the base and the anion from the acid.
For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the following reaction occurs:
HCl + NaOH → NaCl + H2O
In this reaction, the hydrogen ion (H+) from the acid combines with the hydroxide ion (OH-) from the base to form water (H2O). The sodium ion (Na+) from the base combines with the chloride ion (Cl-) from the acid to form sodium chloride (NaCl).
Precipitation Reactions, Acids and bases review worksheet
Precipitation reactions occur when two ions in solution combine to form an insoluble solid precipitate. The precipitate is a compound that is not soluble in the solvent. Precipitation reactions are often used to separate ions from a solution.
For example, when barium chloride (BaCl2) reacts with sodium sulfate (Na2SO4), the following reaction occurs:
BaCl2 + Na2SO4 → BaSO4 (s) + 2 NaCl
In this reaction, the barium ion (Ba2+) from the barium chloride combines with the sulfate ion (SO42-) from the sodium sulfate to form barium sulfate (BaSO4), which is an insoluble solid precipitate. The sodium ion (Na+) from the sodium sulfate combines with the chloride ion (Cl-) from the barium chloride to form sodium chloride (NaCl), which is soluble in water.
Redox Reactions
Redox reactions are reactions that involve the transfer of electrons between atoms or ions. Redox reactions are often used to generate electricity or to produce chemicals.
For example, when iron (Fe) reacts with copper sulfate (CuSO4), the following reaction occurs:
Fe + CuSO4 → FeSO4 + Cu
In this reaction, the iron atom loses two electrons to the copper ion, which gains two electrons. The iron atom becomes oxidized (loses electrons), while the copper ion becomes reduced (gains electrons).
Applications of Acids and Bases: Acids And Bases Review Worksheet
Acids and bases play crucial roles in various aspects of our daily lives. They find applications in industries, households, and even in our bodies.
Let’s explore some of the common applications of acids and bases:
Batteries
- Batteries rely on chemical reactions involving acids and bases to generate electricity.
- Lead-acid batteries, commonly used in cars, contain sulfuric acid (H 2SO 4) and lead (Pb).
- Lithium-ion batteries, found in laptops and phones, utilize lithium ions (Li +) and a combination of acids and bases.
Fertilizers
- Acids and bases are essential components of fertilizers that enhance plant growth.
- Nitric acid (HNO 3) and sulfuric acid (H 2SO 4) are used to produce nitrogen- and sulfur-containing fertilizers.
- Ammonium hydroxide (NH 4OH) and urea (NH 2CONH 2) are nitrogen-rich bases used as fertilizers.
Household Cleaning Products
- Acids and bases are commonly found in household cleaning products due to their ability to dissolve dirt and grime.
- Hydrochloric acid (HCl) is used in toilet bowl cleaners to remove mineral deposits.
- Sodium hydroxide (NaOH) is used in oven cleaners to break down grease and food residue.
- Vinegar (CH 3COOH) is a weak acid used as a natural cleaning agent and disinfectant.
Detailed FAQs
What is the pH scale?
The pH scale is a measure of the acidity or basicity of a solution, ranging from 0 to 14. A pH below 7 indicates an acidic solution, a pH of 7 indicates a neutral solution, and a pH above 7 indicates a basic solution.
What is the difference between an acid and a base?
Acids are substances that donate protons (H+ ions), while bases are substances that accept protons.
What are some examples of acids and bases?
Common acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). Common bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).